Difference between revisions of "Allotrope"
(Created page with "==Key Stage 4== ===Meaning=== Allotropes are different arrangements of atoms in an element. ===About Allotropes=== : Different allotropes of the same elemen...") |
|||
| (2 intermediate revisions by one other user not shown) | |||
| Line 20: | Line 20: | ||
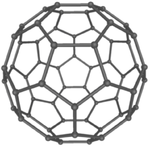
| style="height:20px; width:150px; text-align:center;" |[[Fullerene]]s have a [[Giant Covalent Structure|giant covalent structure]] where each [[Carbon]] [[atom]] has 3 bonds with [[adjacent]] [[atom]]s forming a [[sphere]]. | | style="height:20px; width:150px; text-align:center;" |[[Fullerene]]s have a [[Giant Covalent Structure|giant covalent structure]] where each [[Carbon]] [[atom]] has 3 bonds with [[adjacent]] [[atom]]s forming a [[sphere]]. | ||
|} | |} | ||
| + | |||
| + | ===References=== | ||
| + | ====AQA==== | ||
| + | :[https://www.amazon.co.uk/gp/product/1782945571/ref=as_li_tl?ie=UTF8&camp=1634&creative=6738&creativeASIN=1782945571&linkCode=as2&tag=nrjc-21&linkId=9e29fad914244909903e5e93f8a01d39 ''Allotropes of carbon, page 34, GCSE Chemistry; The Revision Guide, CGP, AQA'] | ||
| + | :[https://www.amazon.co.uk/gp/product/178294639X/ref=as_li_tl?ie=UTF8&camp=1634&creative=6738&creativeASIN=178294639X&linkCode=as2&tag=nrjc-21&linkId=51599bb45a2bfaf7c1b6a978b2ca2616 ''Allotropes of carbon, pages 87-89, GCSE Combined Science Trilogy; Chemistry, CGP, AQA'] | ||
| + | :[https://www.amazon.co.uk/gp/product/1782945962/ref=as_li_tl?ie=UTF8&camp=1634&creative=6738&creativeASIN=1782945962&linkCode=as2&tag=nrjc-21&linkId=476bb5c8d1dfb5c08ac81b6d4d1c98d8 ''Allotropes of carbon, pages 89-91, GCSE Chemistry, CGP, AQA'] | ||
| + | ====Edexcel==== | ||
| + | :[https://www.amazon.co.uk/gp/product/1292120193/ref=as_li_tl?ie=UTF8&camp=1634&creative=6738&creativeASIN=1292120193&linkCode=as2&tag=nrjc-21&linkId=572df39392fb4200db8391d98ae6314e ''Allotropes of carbon, pages 188-189, GCSE Combined Science, Pearson Edexcel ''] | ||
| + | :[https://www.amazon.co.uk/gp/product/1292120215/ref=as_li_tl?ie=UTF8&camp=1634&creative=6738&creativeASIN=1292120215&linkCode=as2&tag=nrjc-21&linkId=8f96ddb76196848bafdb124354e4cf77 ''Allotropes of carbon, pages 44-45, GCSE Chemistry, Pearson, Edexcel ''] | ||
| + | |||
| + | ====OCR==== | ||
| + | |||
| + | :[https://www.amazon.co.uk/gp/product/0198359829/ref=as_li_tl?ie=UTF8&camp=1634&creative=6738&creativeASIN=0198359829&linkCode=as2&tag=nrjc-21&linkId=90e8d7b4f039d53035238fa0320fe00b ''Allotropes, pages 74, 87, Gateway GCSE Chemistry, Oxford, OCR ''] | ||
Latest revision as of 10:26, 30 November 2019
Contents
Key Stage 4
Meaning
Allotropes are different arrangements of atoms in an element.
About Allotropes
- Different allotropes of the same element can have very different physical properties such as melting point and electrical conductivity.
Examples
These are several allotropes of Carbon.
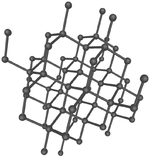
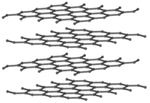
| Diamond is a giant covalent structure where each Carbon atom has 4 bonds with adjacent atoms. | Graphite has a giant covalent structure with each Carbon atom has 3 bonds with adjacent atoms in a layer with loose bonds between the layers. | Graphene has a giant covalent structure where each Carbon atom has 3 bonds with adjacent atoms forming a layer that is one atom thick. | Fullerenes have a giant covalent structure where each Carbon atom has 3 bonds with adjacent atoms forming a sphere. |
References
AQA
- Allotropes of carbon, page 34, GCSE Chemistry; The Revision Guide, CGP, AQA'
- Allotropes of carbon, pages 87-89, GCSE Combined Science Trilogy; Chemistry, CGP, AQA'
- Allotropes of carbon, pages 89-91, GCSE Chemistry, CGP, AQA'
Edexcel
- Allotropes of carbon, pages 188-189, GCSE Combined Science, Pearson Edexcel
- Allotropes of carbon, pages 44-45, GCSE Chemistry, Pearson, Edexcel